H. Rudolph12, M. Kirchhoff2 and S. Gliesmann2
Introduction
Neither the bulk of Sphagnum, which is greater than that of all other bryophyte genera, nor the fact that there is more carbon locked up in Sphagnum than is fixed by all terrestrial vegetation in one year as calculated by Clymo and Hayward (1982), makes Sphagnum peculiar. Rather, it is the economic importance and the increasing ecological, physiological, and biochemical interest in this bryophyte group during the last two decades.
It is our intention to review published and unpublished cultivation techniques, for each experimental approach is limited by the ability to cultivate the mosses under controlled conditions; otherwise one cannot obtain reproducible and comparable results.
The cultivation techniques applied can be divided into three main categories: axenic culture techniques, monocultures in phytotrons, and the cultivation of Sphagnum in model ecosystems under controlled conditions.
Axenic Culture Techniques
Axenic cultures on solid substrates
It is easy to establish axenic cultures of liverworts and mosses on solid substrates (Lai 1984, and references therein). Simon (1988) describes culturing of Sphagnum colonies on solid substrates.
Axenic suspension cultures
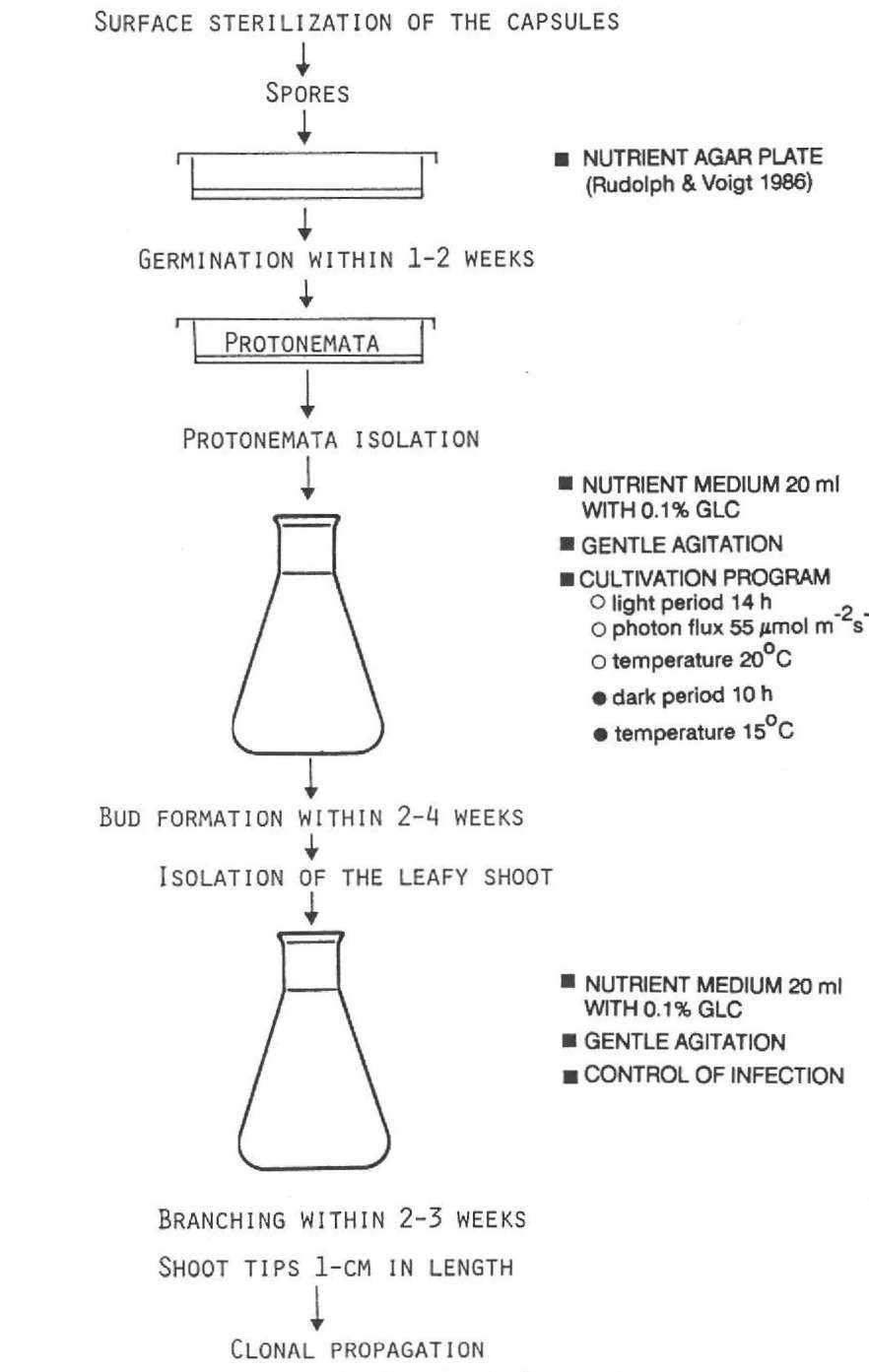
Preparing axenic stock cultures for clonal propagation — Basiie (1972) introduced the method of surface sterilization of small plant parts. Another way to set up axenic suspension cultures is to start with unispore cultures. After surface sterilization of the capsules, the released spores are plated on sterilized agar plates under axenic conditions (Fig. 1). Normally the spores germinate within 1-2 weeks (see Simon 1988). The protonemata are picked and subcultured in a 20 ml standard nutrient solution in 100 ml Erlenmeyer flasks. For this cultivation step the nutrient solution is enriched with 0.1% glucose to get a first test for contamination. Gentle agitation of the flasks is favorable for the development of the inoculum. For such stock cultures we set the following cultivation program: light 14 h, photon flux 55 (μmol m*2 s*1, temperature 20±0.5°C; dark 10 h, 15±0.5°C.
Bud formation appears in 2-4 weeks, depending on the species. As described by Longton and Schuster (1983), Nishida (1970), Clymo and Duckett (1986) and others,
'Author to whom all correspondence should be addressed.
2Botanisches Institut der Christian-Albrechts-Universität zu Kiel, D-2300 Kiel, Biologiezentrum, Otshausenstra/Je 40, FRG.

Figure 1. Routine to prepare axenic cultures for clonal propagation.
the thallose protonemata each produce only one bud. The isolated leafy shoot is transferred onto a new medium and within 2-3 weeks many lateral branches develop. Shoot tips 10 mm long are snipped off and used for clonal propagation.
By this technique we have obtained many clones from different Sphagnum species, including S. fallax (Klinggr.) Klinggr., S. cuspidatum Hoffm., S. majus (Russ.) C. Jens.,
and 5- magellanicum Brid. They all thrive well, and from time to time the medium is checked for possible contamination by incubating aliquots on nutrient agar, nutrient broth, and yeast extract (DIFCO).
Batch cultures — Repeatedly researchers have described that inorganic media are insufficient to provide normal growth and development of Sphagnum in axenic suspension cultures (Simola 1969; Hintikka 1972; Boatman 1978; Jones 1978; Baker & Boatman 1985; Kajita et at. 1987). In non-agitated batch cultures the protonema development, bud formation, and growth of shoots are poor without any addition of an organic carbon source. Simola (1969, 1975, 1979) studied the effect of several sugars, amino acids, and dipeptides on the growth of Sphagnum in axenic cultures. Ca 200-230 mg dry weight were produced in 100 days when one 10 mm shoot tip was used as inoculum per flask containing a nutrient medium with 1% sucrose as a carbon source (Simola 1977).
In general we can confirm these results. Shoot tips (ca 5 mm long) of Sphagnum fallax used as an inoculum grow vigorously when an organic carbon source is added to the nutrient solution. Branching occurs and side shoots are produced in typical patterns (Simon et al. unpub data).
Likewise, when a shoot tip corresponding to a dry weight of 0.7 mg is used as inoculum per flask, the dry weight increases significantly. Flasks containing 50 ml nutrient solution are agitated; the cultivation program is the same as for the stock cultures. We measured the highest dry weight production in a nutrient solution containing 1% sucrose (Fig. 2), with more than 17 mg dry weight produced in 25 days. With 1% glucose the dry weight production is significantly lower than with sucrose. Without any addition of an organic carbon source the dry weight gain is nearly negligible.
Jones (1978) found soluble carbohydrates in inorganic culture solutions containing aseptically cultivated Sphagnum shoots. He concluded that, to grow normally in liquid culture, Sphagnum requires sugars to compensate for those carbohydrates lost by leakage.
Baker and Boatman (1985) showed that the growth of Sphagnum cuspidatum in aqueous solutions is limited mainly by the C02 supply. They contend that in acid nutrient solutions a shortage of C02 will arise since there is no reservoir of bicarbonate. Plants resembling those that occur naturally could be cultivated when air enriched with carbon dioxide was passed through an inorganic nutrient solution. Data concerning the dry weight production under the experimental conditions used are not presented.
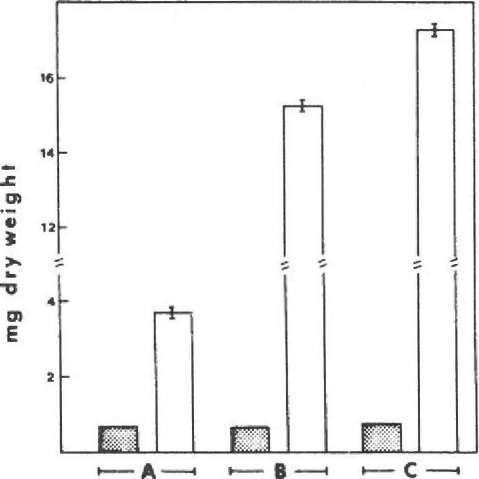
Figure 2. Dry weight production of Sphagnum fallax with additional carbon sources; bars = S. E. Empty bar = dry weight of inoculum; stippled bar = dry weight per flask after 25 days. A. standard nutrient solution; B. standard nutrient solution + 1% glucose; C. standard nutrient solution + 1% sucrose.
The central problems in batch cultures are avoiding the deficiency of C02 and nutrients and the accumulation of metabolic products excreted into the medium.
Katoh et al. (1979) and Katoh (1983) describe photoautotrophic growth of Marchantia polymorpha L. cells in suspension cultures using 1% C02 in air as the sole carbon source. Bopp et al. (1964) and Boatman and Lark (1971) used the cellophane disk technique to supply moss protonemata with fresh nutrient medium; Basile (1964) introduced the technique to remove and replace nutrient medium with syringes. The culture by drip-feeding (fresh liquid medium is dripped directly onto the growing protonemata of mosses such as Physcomitrella patens supported on a nylon net) was described by Cove et al. (1979). An airlift fermenter for the culture of Physcomitrella patens protonemata working with air enriched with 1% C02 was presented at the workshop by Boyd et al. (1988).
It is often a problem in chemical and biochemical studies to have sufficient moss biomass grown under defined axenic conditions. For that reason we developed a continuous feed fermenter that produces high rates of biomass via photoautotrophic growth of Sphagnum plants.
The continuous feed fermenter for the culture of Sphagnum — In principle the technique to improve the nutrient supply and to stabilize the in vitro environment is well known. In comparison to liquid cultures in Erlenmeyer flasks, the continuous medium replacement does provide a significant advantage. The system assures a homeostatic environment in which a supply of nutrients and a constant pH are maintained.
Sophisticated fermenter techniques are used for cell-suspension cultures, microorganisms, and algae. So the way should be open for handling moss plants in the same way as unicellular or filamentous organisms in a chemostat. Multicellular organisms that form larger aggregates are more difficult to deal with in a fermenter, for clumping soon takes place, together with layering. It is more difficult to achieve adequate agitation, especially turbulence, without damaging the branched leafy moss shoots. Therefore common types of stirrers are not appropriate. An airlift (rising current of air) fermenter is specially suited to agitate the extremely delicate moss shoots.
The fermenter body — Figure 3 gives a diagrammatic representation of the complete culturing device. We have obtained useful results with fermenter bodies (3) made from freestanding, wide-necked, globe-shaped flasks of 2 and 6 liter capacity, fitted with an extra neck (4) and a harvest tube (26).
Feed system — Fresh sterile medium (1) is added by a feeding pump (2) to the fermenter body (3). The feed system must be capable of working under sterile conditions (32 & 33 membrane filter). A peristaltic pump such as a Micro Perpex pump (LKB 2132) is the most suitable for feeding. The culture medium volume is kept constant by a level overflow device (5); the effluent pump (6) is set at a higher rate. Clogging of the overflow is prevented by a 230 /im gauze filter (7). The effluent medium is collected in a receiver bottle (8) with an air vent filter (9) so that it is possible to analyze metabolic products excreted by the mosses into the culture medium.
Agitation, gas supply — When cells are grown in a stationary liquid medium the nutrients around them are rapidly depleted. Agitation by rising air bubbles prevent sedimentation and the shoots are well flushed with the nutrient medium. By that technique the fragmentation rate is very low.
Air enriched with 0.5% C02 (10) is first humidified by bubbling it through a column with distilled water (11) held 2°C below the working temperature. The influent gas is filtered by two in-line membrane filters [50 mm, Schleicher & Schiill BA 83(12) and Millex FG50 (13) pore size 0.2 μm], and streams out via a gas distribution tube with a filter disc of sintered glass, porosity P O (14) at the bottom of the fermenter vessel, thus mixing the moss shoots and providing the Sphagnum with C02. To avoid the deposition of water in the air-out line (15) and filter (16), a condenser with a special vapor bulb (Martin-Kugel) (17) is inserted. The actual flow rate (26 1 h-1) of the gas mixture set by a valve (18) is measured with a calibrated flowmeter (Rota) (19).
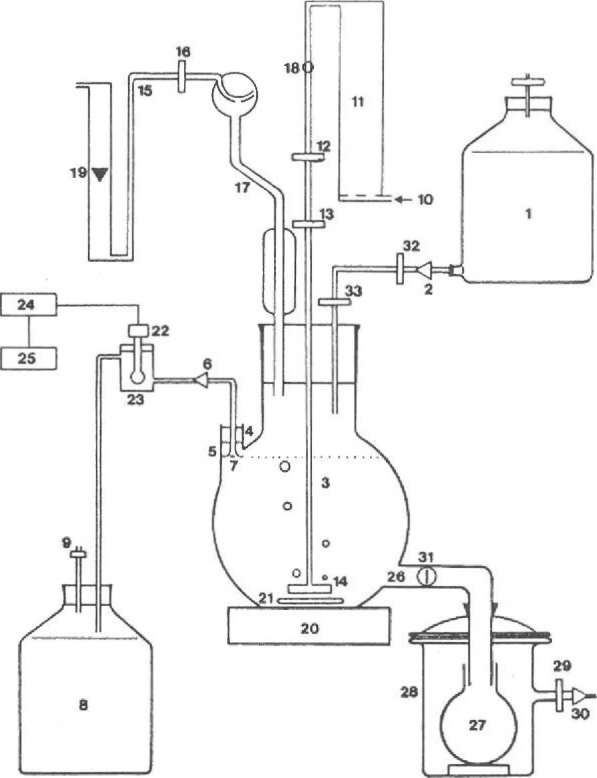
Figure 3. Diagrammatic representation of the essential features of the continuous feed fermenter and the associated equipment. 1. nutrient medium contained in a 5-1 bottle with air-vent filter. 2. feeding pump. 3. fermenter body (nominal capacity 2 or 6 1). 4. extra neck. 5. constant level overflow. 6. effluent medium pump. 7. gauze filter. 8. receiver bottle for the effluent medium. 9. filter. 10. air enriched with 0.5% CO 11. column with distilled water. 12, 13. 0.2 nm membrane filter. 14. gas distribution tube with filter disc of sintered glass, porosity P O. IS. air-out line. 16. filter. 17. cooler and vapor bulb. 18. valve. 19. flowmeter. 20. magnetic stirrer. 21. rotating bar. 22. pH electrode. 23. measuring cell. 24. pH meter. 25. recorder. 26. harvest tube. 27. harvest bottle. 28. attachment with air filter. 29. filter. 30. vacuum pump. 31. valve. 32, 33. 0.2 μm membrane filter.
Sterilization, control of infection — Before setting up, the whole assembly (fermenter body, silicone stoppers, silicone tubing, filter etc.) is sterilized for 20 m in by autoclaving at 121°C. The nutrient medium contained in 5-liter bottles is sterilized twice at 121°C for 60 min. The whole apparatus is reassembled and inoculated under aseptic conditions. With care there should be no risk of contamination so that by suitable precautions pure culture conditions can be maintained. Infection arises from three main causes: failure in sterilization, unsystematic working, and untidiness in air or nutrient filters. The contamination is detected by plating the culture medium to a nutrient agar slope and inoculating into nutrient broth and yeast extract (DIFCO).
Inoculation — Inocula for the fermenter (10 mm shoot tips) are precultivated in culture tubes (50 mm diameter, 500 mm long) containing nutrient medium without any addition of an organic carbon source. Air enriched with 0.5% C02 is passed through the medium. After 10 days of precultivation ca 10 shoots are aseptically transferred into the fermenter body.
Culture conditions — The fermenter is illuminated with two banks of fluorescent tubes, one bank on either side of the culture vessel, with 7 Osram L 65W/25R tubes (photon flux density 105 μmol m-2 s-1, 400-700 nm, determined with a LI-COR quantum sensor). All equipment is in a phytotron at 14 h light, 20±0.5°C, 10 h dark, 15 ±0.5°C.
Feed rate, pH monitoring — The flow rate of the fresh medium depends on the amount of inoculated shoots and the growth rate of the mosses. For flow control we use the pH of the medium monitored by an electrode (22) in a measuring cell (23) positioned behind the effluent pump. Different factors cause acidification of the culture medium, e.g. the continuous production of new cation exchange sites or the resources applied. Usually we cultivate at a pH range of 3.8 - 3.9, feeding a nutrient medium concentrate with a pH of 5.8.
Harvest line — Samples are withdrawn via a harvest tube (26) into a harvest bottle (27). The bottle is kept in an attachment (28) with an air filter (29) in line to the vacuum pump (30). To take a sample, the valve (31) is opened and the material is removed by suction.
Growth of cultures — After inoculation the Sphagnum shoots start growing vigorously. New shoots arise from pre-existing branches or axillary buds adjacent to or some distance from a fascicle of branches. We occasionally observe filamentous and thalloid structures on which buds develop. Detached leaves show the lowest regeneration ability, in general confirming the regeneration studies of Sobotka (1976), Karunen and Kalviainen (1985), and Clymo and Duckett (1986).
The habit of Sphagnum cultivated in that way does not equal that of plants grown under natural conditions. If there is space enough for undisturbed development, the inoculated shoots grow into a ball shape. Sphagnum magellanicum especially has an extreme tendency for spherical growth; at the workshop we demonstrated a ball of 120 mm diameter. The anatomy of the leaf cells is characteristically changed (unpub data).
To increase the biomass production we use regeneration of detached fragments. By activation of a magnetic stirrer (20) via a timer, the rotating bar (21) inside the vessel fragments the branched shoots, so the system has the potential for large-scale production of moss biomass.
The dry weight production of Sphagnum fallax grown for 36 days under photoautotrophic conditions in a 2-liter fermenter is documented in Figure 4. Within the first 30 days after inoculation, no plant material is harvested. During this time the moss tissue produced increases up to 3.8 g dry weight. After harvesting (arrow) the remaining shoots — corresponding to 2.5 g dry weight — produce so much biomass that the content must be reduced (arrow). If the desired steady state is ca 2.5 g, every 4 days ca 1.3 g dry weight must be harvested.
Figure 4. Dry weight production of Sphagnum fallax in a 2-liter fermenter.
Monocultures in Phytotrons
The aim of monocultures is to cultivate several species under controlled conditions so that the mosses maintain their natural habit for many years. The method we describe here has been used in our laboratory for 25 years for special physiological and biochemical research (Rudolph 1963,1978); only the improvements are included.
Container for culturing
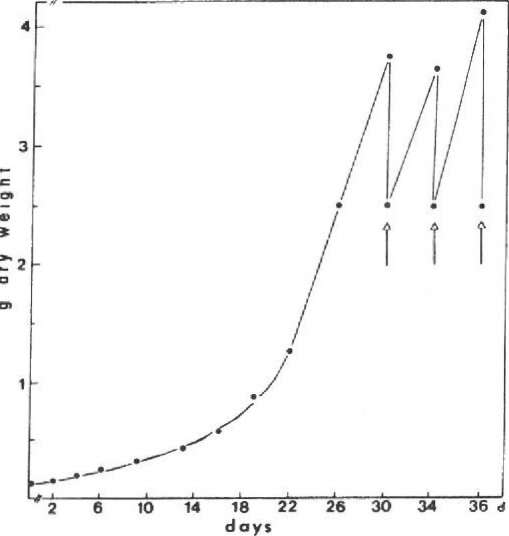
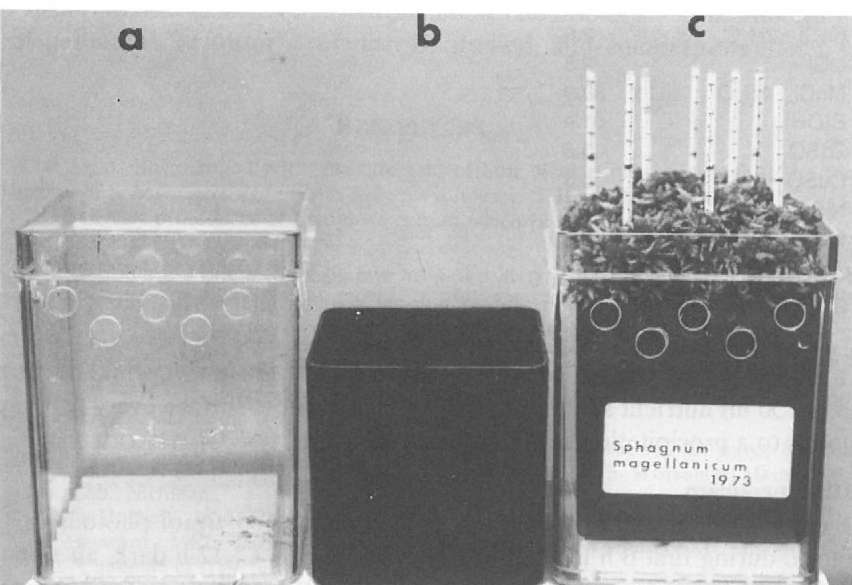
Figure 5. Container for Sphagnum monocultures, a. external box; b. internal container; c. Sphagnum magellanicum with glass rods for growth measurement, cultivated since 1973.
We put 100-120 plants in a plastic container 80 x 80 x 90 mm (Fig. 5), painted black with Inertol 49 W (b) and provided with 5 holes (3 mm) at the bottom. This container is placed on a grate (10 mm high) in an external box, 100 x 100 x 150 mm (a) provided with 5 holes (10 mm) per side to provide ventilation to the upper stem parts (c).
Standard nutrient solution
The nutrient solution applied is in principle the nutrient solution described by Rudolph and Voigt (1986). Because we found that phosphate is a growth-limiting factor (Rudolph unpub data), we enhanced the amount of KH2P04 and used a concentration three times as great. For preparing the standard medium we use the following stock solutions:
|
(NH4)S04 |
6 g/l |
(45 mM) |
(a) |
|
MgSO -7H 0 |
10 g/l |
(40 mM) |
(b) |
|
CaSO A- 2H 20 4 2 |
1.67 g/l |
(9.6 mM) |
(c) |
|
CaCI -2H O 2 2 |
2 g/l |
(14 mM) |
(d) |
|
KH TO 2. 4 |
2 g/i |
(15 mM) |
(e) |
|
KNO 3 |
' 2 g/l |
(20 mM) |
(f) |
|
NH NO i 3 |
5 g/l |
(62 mM) |
(g) |
|
NaOH |
4.4 g/l |
(110 mM) |
<h) |
|
FeCI -6H 0 3 2 |
1 g/l |
(4 mM) + 1.38 g Titriplex III |
(i) |
|
HN03 |
0.12 M |
00 |
|
|
NH 3 3 |
3.6 x 10 |
2M (prepared by ammonia solution 25%, |
|
|
concentration checked by nesslerization) |
(i) |
||
|
trace elements mg/l H 0 (= A-Z solution of Hoagland, 10"1 dilution): |
(m) |
||
|
AI2(S04)3 |
5.5 |
||
|
Kl |
2.8 |
||
|
KBr |
2.8 |
||
|
5.5 |
|||
|
Sndl -2H 0 2 2 |
2.8 |
||
|
UCI |
2.8 |
||
|
MnCI -4H 0 2 2 |
38.9 |
||
|
B(OH) |
61.4 |
||
|
ZnS04 |
5.5 |
||
|
CuS04-5H 0 |
5.5 |
||
|
NiSO -7H D |
5.9 |
||
|
Co(NO ) -6H 0 3 2 2 |
5.5 |
||
10 ml of the stock solutions a, b, d, f, g, h, i, k, I, m, and 30 ml of c and e are added to 20 I distilled water. The ion concentration of the standard nutrient solution (^M) is Na+: 55; 32; NH 95; Ca+Z: 22; Mg+Z: 22; Fe+3: 2; CI" : 20; NO 100; SO '2: 58; PO "3: 22; pH 5.8.
3 4 4
Using a bottle-top dispenser, provided with a 100 x 100 mm sprinkling nozzle, we sprinkle the 50 ml nutrient solution onto the 100 cm2 moss surface every two days. This corresponds to a precipitation of 900 mm per year.
We cultivate Sphagnum at 12 h light, photon flux 105 /imol photons m"2 s"1; air temperature during first 6 h light 21°C, following 6 h 18°C; 12 h dark, air temperature 16.5°C. Due to the radiant flux the temperature in the capitula is ca 2°C higher than the air temperature.
Transpiration accounts for a water loss of 60% under the conditions in the phytotrons (wind velocity in vertical direction ca 0.3 m s"1). The plastic grate
positioned on the bottom of the external container prevents the stems from contacting the liquid. To avoid accumulation and recycling of minerals, we draw off the liquid from the external box by suction.
The growth rate is measured by 10 glass rods calibrated by a mm scale (Rudolph 1963) (Fig. 5c).
Cultivation of Sphagnum in Model Ecosystems under Controlled Conditions
Sphagnum carpets and the microclimate in which they grow create a specific habitat where relatively few other species are able to flourish. Model ecosystems are important for culture of Sphagnum together with vascular plants and other bryophytes. To set up such cultures, pieces of naturally growing bog vegetation are planted in a container (600 mm x 400 mm x 120 mm). Each container can be drained via a valve. Ten-fifteen containers are placed together on a table in an air-conditioned greenhouse. In spring and summer the moss system should be cultured at 21°C air temperature in light, 10°C dark; relative humidity 85-90%; wind velocity ca 0.5 m s-1. The roof is automatically shaded when light intensities increase to 30,000 lux. In autumn and winter we use additional irradiation with Osram 400 W HQI-TS lamps (irradiance 30 W m-2), light 12 h minimum. The temperature during these seasons is reduced to 10-15°C day, 2-5°C at night. For mineral supply the bog surface is sprinkled once a day with standard nutrient solution corresponding to a precipitation of 900 mm per year.
We have cultivated such model ecosystems for more than 5 years with good results, producing a homogeneous carpet of Sphagnum with no gaps. In such a system the effects of experimental changes in environmental conditions such as mineral supply, addition of pollutants, or water stress can be studied, and competition effects can be analyzed.
Baker, R. G. & D. J. Boatman. 1985. The effect of carbon dioxide on the growth and vegetative
reproduction of Sphagnum cuspidatum in aqueous solutions. J. Bryol. 13: 399-406. Basile, D. V. 1964. New procedures of bryophyte culture which permit alteration of the culture medium during the life cycle. Bryologist 67: 141-146.
-. 1972. A method for surface-sterilizing small plant parts. Torreya 99: 313-316.
Boatman, D. J. 1978. Experiments on the growth of protonemata of Sphagnum papillosum. Bull. Br. Bryol. Soc. 3:10.
--& P. M. Lark. 1971. Inorganic nutrition of the protonemata of Sphagnum papillosum Lindb.,
Sphagnum magellanicum Brid. and Sphagnum cuspidatum Ehrh. New Phytol. 70: 1053-1059. Bopp, M., H. Jahn & B. Klein. 1964. Eine einfache Methode, das Substrat wahrend der Entwicklung von
Moosprotonemen zu wechseln. Rev. Bryol. Lichdnol. 33: 219-223. Boyd, P. J., J. Hall, & D. J. Cove. 1988. An air-lift fermenter for the culture of the moss, Physcomitrella patens. In J. M. Glime (ed.): Methods in bryology. Proc. Bryol. Meth. Workshop, Mainz. Pp. 41-45. Hattori Bot. Lab., Nichinan. Clymo, R. S. & J. G. Duckett. 1986. Regeneration of Sphagnum. New Phytol. 102: 589-614.
--- & P. M. Hayward. 1982. The ecology of Sphagnum. In A. J. E. Smith (ed.): Bryophyte ecology.
Pp. 229-290. Chapman & Hall, New York. Cove, D. J., N. W. Ashton, D. R. Featherstone & T. L. Wang. 1979. The use of mutant strains in the study
of hormone action and metabolism in the moss Physcomitrella patens. Proc. 4th John Innes: 231-241. Hintikka, V. 1972. Variation in gametophyte morphology of Sphagnum fallax in aseptic culture. Ann. Bot. Fenn. 9: 91-%.
Jones, D. G. 1978, Aspects of growth and development in Sphagnum cuspidatum. Bull. Br. Bryol. Soc. 3: 8-10.
Kajita, M., S. Takio, S. Tafcami & S. Hino. 1987. Establishment and growth characterization of suspension culture of cells from the moss, Sphagnum imbricatum. Physiol. Plant. 70: 21-26.
Karunen, P. & E. Kälviäinen. 1985. Senescence and post-mortem changes in the ultrastructure of Sphagnum Juscum (Klinggr.) Schleich leaf cells. New Phytol. 100: 419-427.
Katoh, K. 1983. Kinetics of photoautotrophic growth of Marchantia pofymorpha cells in suspension culture. Physiol. Plant. 59: 242-248.
-, Y. Ohta, Y. Hirose & T. Iwamura. 1979. Photoautotrophic growth of Marchantia pofymorpha L.
cells in suspension culture. Planta 144: 509-510.
Lai, M. 1984. The culture of bryophytes including apogamy, apospory, parthenogenesis and protoplasts. In A. F. Dyer & J. G. Duckett (eds.): The experimental biology of bryophytes. Pp. 97-115. Academic Press, London.
Longton, R E. & R M. Schuster. 1983. Reproductive biology. In R M. Schuster (ed.): New manual of bryology. Pp- 386-462. Hattori Bot. Lab.
Nishida, Y. 1970. Studies on the differentiation of the protonema in two species of Sphagnaceae. Bot. Mag. Tokyo 83: 249-253.
Rudolph, H. 1963. Die Kultur von Hochmoor-Sphagnen unter definierten Bedingungen. Beitr. Biol. Pflanz. 39: 153-177.
-. 1978. 15 Jahre Kultur von Sphagnen unter definierten Bedingungen: Eine Übersicht über
Resultate, Probleme und Perspektiven. Bryimhytorum Bibl. 13. Pp. 297-309. J. Cramer, Vaduz.
-& J.-U. Voigt. 1986. The effects of NH -N and NO "-N on growth and metabolism of Sphagnum
magellanicum. Physiol. Plant. 66: 339-343. 4
Simola, L. K. 1969. The effect of various mono- and disaccharides on the growth of Sphagnum nemoreum thalli in sterile cultures. Physiol. Plant. 22:1079-1084.
-. 1975. The effect of several protein amino acids and some inorganic nitrogen sources on the
growth of Sphagnum nemoreum. Physiol. Plant. 35: 194-199.
-. 1977. The tolerance of Sphagnum fimbriatum towards lead and cadmium. Ann. Bot. Fenn. 14:
1-5.
-. 1979. Dipeptide utilization by Sphagnum fimbriatum. J. Hattori Bot. Lab. 46: 49-54.
Simon, E. 1988. Axenic cultures of Sphagnum on solid substrates. In J. M. Glime (ed.): Methods in bryology. Proc. Bryol. Meth. Workshop, Mainz. Pp. 35-40. Hattori Bot. Lab., Nichinan.
Sobotka, D. 1976. Regeneration and vegetative propagation of Sphagnum palustre as a factor of stability. Acta Soc. Bot. Pol. 25: 357-368.
axenic cultures of sphagnum on solid substrates
Introduction
Investigations concerning associations between Sphagnum and other organisms such as fungi or Mycobacteria (Untiedt & Mueller 1985; Simon 1987) require cultures of Sphagnum that satisfy the following demands:
The plants have to be sterile so that any changes observed in the Sphagnum after inoculation can reliably be considered as due to the microorganisms under investigation.
The Sphagnum plants should resemble those in their natural habitat as closely as possible in order to rule out any effect on the association caused by uncommon histology or morphology of the peat mosses.
The vigor of the heterotrophic organism must not be influenced by an additional supply of nutrition; neither the substrates nor the nutrient solutions used are allowed to contain any organic substances that might serve as a carbohydrate source for the heterotrophic partner of the association. For these reasons the Sphagnum plants should be grown from spores under axenic conditions on solid substrates.
Materials and Methods
Capsules can be obtained from the field by collecting capitula with mature capsules and placing them in sterile dishes. In the laboratory the pseudopodia are cut and the capsules are collected in sterile dishes. The surface of the capsule is sterilized by dipping the capsules in Bazillol or in 70% (v/v) methanol. The wet capsules are placed in open, sterile glass tubes and dried in the air of a clean bench. During the drying process ripe capsules burst and release the spores. The spores can either be immediately inoculated onto nutrient agar in sterile Petri dishes or can be stored at 4°C in the dark.
Germination time
For the preparation of nutrient agar I have tested Beijerinck solution, as suggested by Anderson and Crosby (1965), and the nutrient solution by Rudolph and Voigt (1986). Though the nutrient solutions differ remarkably in their ionic concentrations, germination rate and time needed for germination are quite the same on both nutrient agars (Table 1). On the other hand, within each set of experiments the periods of time needed for germination vary from five days to two months, not only between different experiments but also even within one experiment.
A survey of the literature shows even more contradictory observations:
'university of Kiel, Botany Department, 2300 Kiel, FRG.
As can be seen in Table 1 the periods of time needed for germination vary from 3 days to half a year. The nutrient solutions applied differ remarkably, but even if two authors use the same nutrient solution they may find different germination times.
Boatman and Lark (1971) point out that a high concentration of phosphate is necessary for germination and development of the sporeling. Thus they ascribe the failure of development of the sporelings in bog water to its low phosphate content that is usually 100 to 1000 fold lower than that of the nutrient solution applied by these authors. On the other hand, Clymo and Duckett (1986) found Sphagnum plants growing from spores on peat slices in the laboratory under a moist atmosphere, and my own investigations show that germination and development of the protonemata occurs as well on phosphate-poor nutrient agar, such as that based on the solution by Rudolph and Voigt (1986), as they do on phosphate-rich agars.
Table 1. Composition of nutrient solutions and corresponding germination times. Data taken from literature as indicated. 1. Anderson & Crosby 1965; 2. Barker & Boatman 1985; 3. Boatman & Lark 1971; 4. Bold 1948; 5. Hintikka 1972; 6. Nishida & Saito 1961; 7. Noguchi 1958; 8. Simon, unpub data.
|
RUDOLPH С VOIGT |
BSIJERINCK |
BOATMAN & LARK |
HINTIKKA |
knop |
benecke |
|
|
Nit (u«) no; (>jm) Mg'4 (pM) Ca" (pn) K* (>lM) Na+ (pM) soj* (>|N) ci* (>JM) H,pct; (jiM) |
95 100 22 22 17 55 57 20 в |
6250 6250 311 fiflO 1470 811 1300 1470 |
4000 913 1000 2000 1333 913 + 1333 |
1870 406 735 406 1670 735 |
11086 576 4800 2450 576 1044 |
|
|
trace-elements |
Fe f Al J , Br Ti ,Sn LI ,Mn В , Zn CU.Ni ,CO |
Fe ,МП 3 t Zn Cu , Mo |
Fe ,Mn В i Zn Cu ,Mo |
Fe |
||
|
glucoss |
- |
- |
- |
0.5 » (w/v) |
- |
|
|
pH |
5.8 |
5.5 |
5.1 |
4.3-4.6 |
||
|
time needed Cor germinat ion |
5-10 d to 2 months (8) |
5-10 d to 2 months (8) 10-15 d (1) |
7 d (2,3) |
1/2 У (5) |
3-5 d (i;4) |
7-10 d (6) 2 months (7) |
Considering these facts it seems most likely that parameters other than the composition of the nutrient solution would affect the germination and the development of the sporelings. First, the germinating Sphagnum spores are much larger than the spores that are released from the capsule. That means that the spores have to take up a considerable amount of water by imbibition before germination. For this reason sufficient water has to be provided. On the other hand, spores should not be submerged because this leads to slow growth, and no thalloid protonemata form. To provide the spores with a suitable amount of water, the spores are suspended in autoclaved water, and 100 jul aliquots of this suspension are placed dropwise onto the agar surface as indicated in Figure 1.1. One ml of spore suspension per Petri dish is
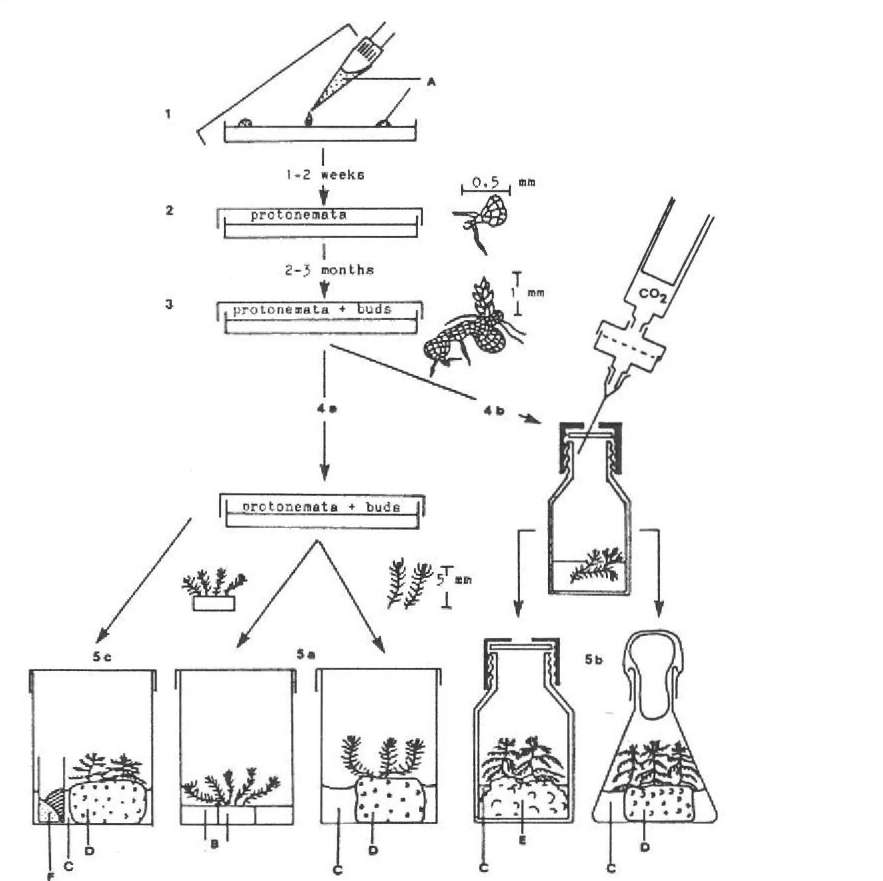
Figure 1. Sequence in establishing axenic culture of Sphagnum, A. spores suspended in water. B.
nutrient agar. C. nutrient solution. D. foam rubber. E. cotton wool. F. glass tube with NaHCO and
CaQ . 3
2
convenient. The Petri dishes are sealed and the lids are fixed with parafilm to prevent drying.
The second parameter I consider most important for germination and development of the protonemata is a suitable illumination. The following experiment shows the dependence of germination and development of the sporeling on different nutrient agars on the one hand and on light intensity on the other (Table 2).
Three nutrient agars were used — those of Boatman and Lark (1971), of Beijerinck (Anderson & Crosby 1965), and of Rudolph and Voigt (1986), the latter varied as to its pH and its phosphate content.
To vary the light intensity, the Petri dishes were piled up in stacks of five. Thus the spores in the top dish were exposed to the full light of the room (180 jUmol m"2 s"1). The spores in the dishes below were consequently exposed to lower light intensities measuring 64, 48,40, and 34% of the full light, respectively.
The development of the protonemata was recorded 10, 25, and 50 days after inoculation. The capital letters in Table 2 correspond to the stage of development as indicated in Figure 2.
The results do not show any severe differences in development of protonemata in cultures with different nutrient agars. By contrast, the light intensity shows a substantial effect on the development of the sporelings. Spores that had been exposed to the full light had in no case developed further than the 1-cell stage of the protonema.
In shaded cultures, however, — especially if the light intensity is restricted to less than 50% — the development of the protonemata is rapid and buds form within 50 days after inoculation.
These results lead to the conclusion that germinating spores and young protonemata should be sheltered from too much light by covering the cultures with white paper.
The cultures are kept in a phytotron at 20°C during the 12 h illumination period and at 5°C in the dark period, but they can also be kept in a laboratory at room temperature. As soon as buds have developed the cultures should be exposed to the full light of the room to accelerate the growth of the leafy gametophyte.
The plants can be grown in the Petri dishes until they have reached a height of 5 to 10 mm (Fig. 1.4a). Then they should be transferred into taller culture vessels containing either new nutrient agar or different substrates such as polyurethane
foam mats or cotton-wool soaked in nutrient deveiopment of Sphagnum fallax. Scalebar solution (Fig. 1.5a). equals 100 jtm.
This method requires little effort but the mosses grow slowly and they show the typical pattern of Sphagnum branching only after a very long culture period. Growth and formation of side branches can be accelerated by providing the cultures with NaHC03 as a C02 source. For this purpose a small open glass tube containing solid NaHC03 and CaCl^, both substances autoclaved separately, is placed into the culture vessel as indicated in Figure 1.5c. Carbon dioxide is released according to the following formula:
2NaHC03 + CaCI2—> 2Na+ + 2CI" + H20 + CaC03 + C02.
Cultures in which the mosses grow faster and resemble Sphagnum in its natural habitat very well can be maintained employing the following method. Thalloid protonemata, carrying buds and having been grown from spores on nutrient agar as usual, are cultivated in liquid culture as described by Rudolph (1988). To spare expensive equipment, the liquid culture may as well be performed as indicated in Figure 1.4b. The plants are grown in culture vessels containing a 10-fold concentrated nutrient liquid used by Rudolph and Voigt (1986). The culture vessel is sealed either with a rubber stopper or with a perforated lid with an additional rubber washer. Carbon dioxide can be injected into the culture vessel by means of a pipetting syringe
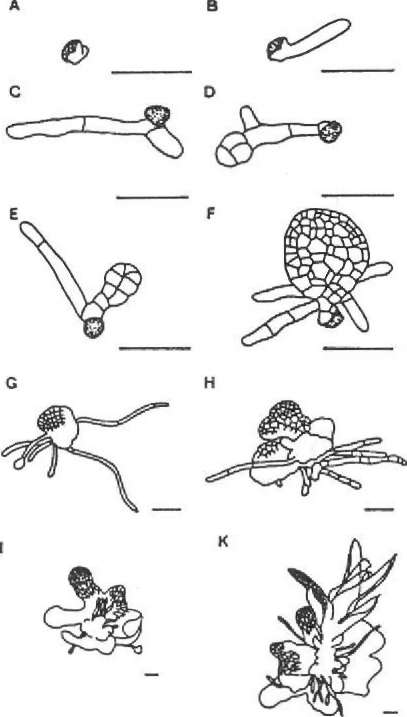
Figure 2. Stages of protonema!
equipped with a membrane filter holder (membranes with 0.2 μm pore size) and a hypodermic needle as indicated.
Table 2. Dependence of^gepnination and protonemata development on nutrient agar and light intensity (100% = 180 ftmol m s ) for Sphagnum fallax. The capital letters correspond to the stages of protonemal development as indicated in Figure 2; 0 = no germination.
|
time (d) |
after inoculation |
|||
|
nutrient agar |
light («) |
10 |
25 |
50 |
|
BOATMAN t LARK |
100 |
0—A |
A-<b| |
a— ( b ) |
|
64 |
a-b-(c) |
(b)-c-(d) |
(b)-c-(d) |
|
|
40 |
D-C |
c-(d> |
(C)-F-C-H-(I) |
|
|
40 |
B-C |
C-(D) |
(g-h)-i-k |
|
|
34 |
c |
c-D |
(g-h)-i-k |
|
|
beijerinck |
100 |
0-a |
a-b |
a |
|
64 |
b-(c) |
B-C |
C-8-F-G-H |
|
|
40 |
b-C |
c-d |
I-K |
|
|
40 |
(B)-c |
(c)-d-(e) |
(G-H)-l-K |
|
|
34 |
C-D |
(C)-D-e-(f |
) Il-(K) |
|
|
rudolph t voigt |
100 |
a |
a-b |
a-b |
|
pH 4 |
64 |
C-0 |
c—e-(f) |
i-k |
|
4P |
c-d |
(c)-e-f |
i 1 -k |
|
|
40 |
c-d |
(C)-e-F |
I-KI |
|
|
34 |
c-d |
(C-E)-F |
i ! -k |
|
|
rudolph & voigt |
100 |
a |
a-b |
a-b |
|
pH 4, with |
64 |
a-b |
c- {d ) |
I-K |
|
additional |
48 |
b-c-d |
(c)-e-f |
I-KI 1 |
|
h,por (1500 um) |
40 |
B-C-D |
(c)-e-f |
1-M |
|
2 I, |
34 |
c-d |
(c-e)-f |
1-ki i |
|
rudolph t voist |
100 |
a |
a-b |
a |
|
pH 5.4 |
64 |
a-b |
(c)-e-f |
I-* |
|
4P, |
a-b |
(C)-E-P |
i-k |
|
|
40 |
a-b-c-d |
(c-e)-f |
i-k |
|
|
34 |
(a-b—c) -d |
<e)-f |
I-K |
|
|
rudolph i voigt |
100 |
a-s |
a |
a-b |
|
pH 5.4, with |
64 |
a-b |
b-c |
b-c |
|
additional |
48 |
b-c-(d) |
(c)-e-f |
i-k |
|
h,p0: (1500 un) |
40 |
b-c-d |
(c)-e-f |
i-ki 1 |
|
34 |
b-c-d |
(c)-e-f |
i-ki |
|
As soon as leafy gametophytes with side branches have developed, they should be transferred onto solid substrates soaked in nutrient solution as described above (Fig. 1.5b). These final cultures again can be supplied with carbon dioxide, either with the help of a pipetting syringe or by inserting a small vessel with NaHC03 and CaC^.
Of course the nutrient liquid should be changed from time to time according to the liquid volume in the vessel and the nutrient solution chosen. I prefer the nutrient solution by Rudolph and Voigt (1986) because it resembles bog water very closely. However, due to its low ionic concentration it has to be changed quite often. The decision as to which nutrient solution, which substrate, and what kind and size of culture vessels have to be applied is left to the experimenter according to the kind of experiments being done.
References
Anderson, L. E. & M. R. Crosby. 1965. The protonema of Sphagnum meridense (Hampe) C. Muell. Bryologist 68: 47-54.
Barker, R G. & D. J. Boatman. 1985. The effect of carbon dioxide in the growth and vegetative
reproduction of Sphagnum cuspidatum in aqueous solutions. J. Bryol. 13: 399-407. Boatman, D. J. & P. M. Lark. 1971. Inorganic nutrition of the protonemata of Sphagnum papillosum
Lindb., S. magellanicum Brid. and S. cuspidatum Ehrh. New Phytol. 70:1053-1059. Bold, H. C. 1948. The prothallium of Sphagnum palustre L. Bryologist 51: 55-63. Clymo, R S. & J. G. Duckett. 1986. Regeneration of Sphagnum. New Phytol. 102:589-614. Hintikka, V. 1972. Variation in gametophyte morphology of Sphagnum in pure culture. Ann. Bot. Fennici 9: 91-96.
Nishida, Y. & S. Saito. 1961. Studies of the germination of the spore of some mosses. II. Diphyscium fitlvifolium Mitt, and Sphagnum cuspidatum Ehrh. Bot. Mag. Tokyo 74: 91-97.

Noguchi, A. 1958. Germination of spores in two species of Sphagnum. J. Hattori Bot. Lab. 19: 71-75. Rudolph, H., M. Kirchhoff & S. Gliesmann. 1988. Sphagnum culture techniques. In J. M. Glime (ed.): Methods in biyology. Proc. Bryol. Meth. Workshop, Mainz. Pp. 25-34. Hattori Bot. Lab., Nichinan,
-& J. U. Voigt. 1986. Effects of NH -N and NO "-N on growth and metabolism of Sphagnum
magellanicum. Physiol. Plant. 66: 339-343. Simon, E. 1987. Zur Biologie sphagnicoler Basidiomyceten. Ph. D. thesis, University of Kiel, FRG. Untiedt, E. & K. Mueller. 1985. Colonization of Sphagnum cells by Lyophyllum palustre. Can. J. Bot. 63: 757-761.